Synthesis, Reactions and Medicinal Uses of Pyridine
Synthesis, Reactions, and Medicinal Uses of Pyridine
Pyridine is a six-membered heterocyclic compound that contains one Nitrogen as heteroatom.
Physical properties:
Colour: colourless
State: liquid
Melting point: -41.6 oC
Boiling point: 115.2 oC
Solubility: Soluble in water
Structure and Aromaticity:
Pyridine contains five carbon atoms and one nitrogen atom, all are sp2 hybridized. Pyridine is a planar molecule that contains unhybridised p orbitals perpendicular to the ring. these p orbitals sidewise overlap to form pi bonds. Pyridine contains a total of six delocalised pi electrons, following the Huckls rule hence pyridine is aromatic.
Resonance structures:
Basicity Of Pyridine:
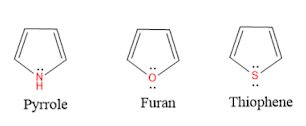
Pyridine is more basic than pyrrole and less basic than Aliphatic amine. In pyridine, lone pair of electrons on nitrogen is easily available for protonation and participate in acid-base reactions. whereas in pyrrole, lone pair of electrons on nitrogen atom gets involved in pyrrole aromaticiy and is not available for acid-base reactions.
When compared with aliphatic amines, Pyridine nitrogen atom sp2 hybridised whereas aliphatic amine sp3 hybridised. sp2 hybridised nitrogen atom more electronegative than sp3 hybridised aliphatic nitrogen. Hence sp2 hybridised nitrogen atom tightly held lone pair electrons and are not easily available for acid-base reaction in comparison with the sp3 hybridised nitrogen atom, It is less electronegative and have loosely held lone pair of electrons and is easily available for acid-base reactions.
Synthesis of Pyridine:
1. Hantzsch Synthesis:
Condensation of two moles of β-dicarbonyl compounds or β-keto esters with aldehyde and ammonia gives substituted pyridine.
2. From Acetylene:
Two moles of Acetylene and Hydrogen cyanide passed through a red hot tube gives Pyridine.
3. Guareschi Synthesis:
Condensation of Acetoaacetic esters and Cyanoacetamide gives Pyridine derivatives.
4. From 1,5-Dicarbonyl compounds:
1,5-Dicarbonyl compounds react with ammonia to give 1,4-dihydropyridines which upon dehydrogenated to give pyridine.
Reactions:
Electrophilic addition at "N" atom:
1. Protonation:
Pyridine reacts with acids gives pyridinium salts.
2. N-Alkylation:
Pyridine reacts with alkyl halides gives N-alkyl pyridinium salts.
Electrophilic substitution reactions:
Electrophilic substitution reactions preferably take place at the 3rd position.
1. Nitration:
Pyridine upon nitration gives 3-nitropyridine.
2. Sulphonation:
Pyridine sulphonation with concentrated sulfuric acid in Mercuric sulfate give pyridine-3-sulphonic acid.
3. Halogenation:
Pyridine upon halogenation gives 3-halo pyridine.
Friedel Craft's reactions are not observed due to aluminium chloride forming complex with pyridine which is highly unreactive.
Nucleophilic substitution reactions:
Nucleophilic substitution reactions in pyridine preferably take place at the 2nd position.
1. Chichibabin reaction:
Pyridine reacts with Sodamide in liquid ammonia gives 2-amino pyridine.
2. Reaction with Alkyl Lithium:
Pyridine reacts with alkyl lithium gives 2-pyridyl lithium.
3. Reaction with KOH:
pyridine reacts with KOH gives 2-hydro pyridine.
Reduction:
1. Pyridine reacts with hydrogen and palladium as catalyst gives Piperidine.
2. Birch reduction: Pyridine reacts with sodium in liquid ammonia gives 1,4-dihydropyridine.
3. Pyridine reduced with Lithium aluminium hydrate gives 1,2-dihydropyridine.
Oxidation:
Pyridine is oxidised with various oxidising agents and gives Pyridine-N-Oxide.
Pyridine as Acylating agent: Pyridine is used as a catalyst for Acyalating phenols, alcohols and amines by reacting with acid chlorides or anhydrides.
Medicinal uses:
Drugs containing Pyridine ring:
1. Isoniazid and Ethionamide: These are used for the treatment of Tuberculosis(TB).
2. Sulfasalazine: Sulfasalazine is used to treat Rheumatoid arthritis.
3. Sulfapyridine: Sulfapyridine is an antibiotic used to treat skin infections.
4. Omeprazole and Lansoprazole: These are proton pump inhibitors used to treat the Peptic ulcer.
5. Vitamin B3: It is also known as Niacin an essential nutrient used to lower bad cholesterol (LDL) and increase good cholesterol (HDL).
6. Vitamine B6: Improve brain health and reduce symptoms of depression and also used to anaemia and peripheral neuropathy.
References (Latest editions):
Heterocyclic chemistry by Raj K. Bansal.
Heterocyclic chemistry by T.L. Gilchrist.
Organic chemistry by Morrison and Boyd.
A textbook of organic chemistry - Arun Bahl. B.S. Bahl.

































Comments
Post a Comment