Synthesis, Reactions and Medicinal Uses of Oxazole
Synthesis, Reactions, and Medicinal Uses of Oxazole
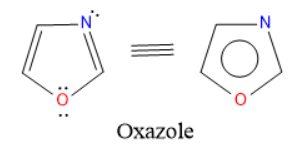
Oxazole is a five-membered heterocyclic compound having one oxygen and one nitrogen atom as a heteroatoms at 1 and 3 positions respectively.
Physical properties:
Colour : clear to pale yellow
State : liquid
Boiling point : 69 to 70oC
Melting point : -84oC
Solubility : partially soluble in water, soluble in organic solvents
Structure & Aromaticity:
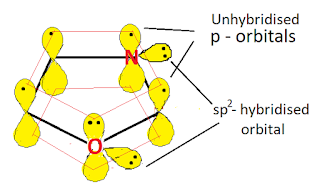
C, N and O atoms in the oxazole ring are sp2 hybridised, so the oxazole contains a planar ring structure. the sp2 hybrid orbitals overlap with each other and with "s" atomic orbital of the three hydrogens forming C-C, C-N, C-H, and C-O sigma bonds. all these sigma bonds lie in one plane.
Oxazole also has unhybridized p orbitals and these are perpendicular to the plane of the ring. each p orbital on carbon atom contains one electron and the p orbitals on nitrogen and oxygen contain lone pair of electrons(two electrons). the p orbitals overlap to form delocalized pi molecular orbital. Oxazole shows aromaticity because the resulting pi molecular orbital (which contain 6 electrons) satisfies the Huckel's rule (n=1 in 4n+2).
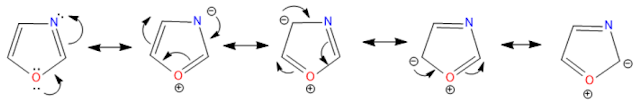
Resonance structures:
Synthesis:
1. Robinson-Gabriel synthesis:
It is the most method for synthesis of oxazoles. It involves an α-acylamino ketone which undergoes cyclisation and dehydration in the presence of phosphorus pentoxide or strong mineral acid. This method is especially applicable for synthesis 2,5-diaryl oxazoles.
Mechanism:2. From Isocyanides: Reaction of α-metallated isocyanides with acid chlorides or anhydrides yields substituted oxazoles.
3. From α-hydroxy carbonyl compounds: α-hydroxy carbonyl compounds condensed with amide or amide derivatives gives substituted oxazoles.Electrophilic substitution reactions:
Oxazole less reactive for electrophilic substitution reactions because of highly electronegative oxygen present in the ring, it makes deactivates the ring and not available for electrophilic substitution reactions. However, if ring activated by electron-donating groups electrophilic substitution reactions are possible at 5th position.
1. Halogenation:
2. Nitration: Nitration and Sulphonation reactions are more difficult on oxazole ring.
Other important reactions:
Protonation: Oxazole acts as a base, accepts proton and forms oxazolium salt.
Reduction: Oxazole forms oxazolidines, when reduced with sodium metal in ethanol.
Oxazole ring is cleaved when reduced with Lithium aluminium hydride.
Oxidation: Oxazole ring is not stable towards oxidising agents, the ring is opened.
N-alkylation: Oxazole reacts with alkyl halides to form quaternary ammonium salts.
Diels-Alder reaction: Oxazole acts diene, undergoes cycloaddition reaction at 2,5- positions.
Synthesis of furan: Cycloaddition of oxazole with alkyne derivative gives furan derivative.
Synthesis of pyridine: (Kondrat'eva pyridine synthesis)
Cycloaddition of oxazole with alkene derivative gives pyridine derivative.
1. Oxaprozin: Used to treat pain, inflammation and rheumatoid disease.
2. Bengazoles: Bengazoles are antifungal agents used to treat fungal infections.
3. Benoxaprofen, Flunoxaprofen: These are analgesic, antipyretic and anti-inflammatory agents used to treat pain due to rheumatoid arthritis and osteoarthritis.
4. Ditazole: It is a non-steroidal anti-inflammatory agent, also have analgesic and antipyretic properties.
5. Aleglitazar: Used in the treatment of diabetes and cardiovascular diseases.
References (Latest editions):
Heterocyclic chemistry by Raj K. Bansal.
Heterocyclic chemistry by T.L. Gilchrist.
Organic chemistry by Morrison and Boyd.
A textbook of organic chemistry - Arun Bahl. B.S. Bahl.



























Comments
Post a Comment