Synthesis, Reactions and Medicinal Uses of Thiazole
Synthesis, Reactions, and Medicinal Uses of Thiazole
Thiazole is a five-membered heterocyclic compound having one Sulphur and one Nitrogen atom as heteroatoms at 1 and 3 positions respectively.
Colour : Colourless or pale yellow
State : liquid
Boiling point : 116 - 118 oC
Melting point : -33 oC
Solubility : slightly soluble in water, soluble in organic solvents
Structure & Aromaticity:
C, N and S atoms in the thiazole ring are sp2 hybridised, so the thiazole contains a planar ring structure. the sp2 hybrid orbitals overlap with each other and with the "s" atomic orbital of the three hydrogens forming C-C, C-N, C-H, and C-S sigma bonds. all these sigma bonds lie in one plane.
Thiazole also has unhybridized p orbitals and these are perpendicular to the plane of the ring. each p orbital on carbon atom contains one electron and the p orbitals on nitrogen and sulphur contain lone pair of electrons(two electrons). the p orbitals overlap to form a delocalized pi molecular orbital. Thiazole shows aromaticity because the resulting pi molecular orbital (which contain 6 electrons) satisfies Huckel's rule (n=1 in 4n+2).
Resonance structures:
Synthesis:
1. Gabriel synthesis: α-Acyl amino ketones undergo cyclization in presence of phosphorous pentasulfide and strong mineral acids like hydrochloric acid or sulphuric acid followed by dehydration to give thiazole.
2. Hantzsch Thiazole synthesis: Condensation of α-halo ketones and thioamides gives thiazole.
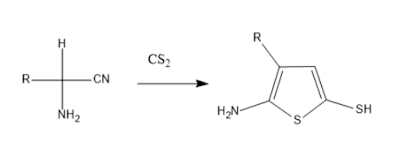
3. From α-aminonitrile: Condensation of α-aminonitrile and carbon disulfide to give thiazole.
4. From thiocyanate salts: halo acetones reacts with thiocyanate to give thiazole.
Electrophilic aromatic substitution reactions:
The pi-electron density is more at C-5 and C-4. In the presence of electron-donating groups, the electrophilic substitution takes place preferably at C-5 followed by C-4.
Suphonation: Thiazole upon sulphonation give Thiazole-5-sulphonic acid.
Halogenation: 2-amino thiazole is treated with bromine in acetic acid gives 5-substituted thiazole.
Nitration: Thiazole upon nitration gives 5-nitro and 4-nitro substituted thiazole in a 2:1 ratio.
2,5 disubstituted thiazole upon nitration gives 4-nitro substituted thiazole.
Nucleophilic substitution reactions:
In thiazole Nucleophilic substitution reactions take place at the C-2 position.
1. Thiazole is reacted with sodamide in liquid ammonia to give 2-amino thiazole.
2. 2-chloro thiazole reacts with sodium methoxide to give 2-methoxy thiazole.
Other reactions:
Protonation: Thiazole acts as the base, when reacts with acids it abstracts the proton to form thiazolium cation.
N-alkylation: Thiazoles reacts with alkyl halides to gives N-alkyl thiazolium salts.
Reduction: Thiazole shows resistance towards many reducing agents. But Raney nickel reduces it and opens the ring.
Oxidation: Thiazole oxidised to give thiazole-N-oxide.
Medicinal uses:
Drugs containing Thiazole ring:
1. Famotidine: Fomatidine is an H-2 receptor blocking agent used in the treatment of peptic ulcers.
2. Thiabendazole: Thiabendazole is an anthelmintic used to treat infections caused by threadworms, hookworms, pinworms and whipworms.
3. Sulphathiazole: Sulphathiazole is an antibiotic. it is used in combination with other sulphonamide antibiotics to treat vaginal infections.
4. Vitamine B1: Used in thiamine deficiency.
5. Fanetizole: Fanetizole is an anti-inflammatory agent used to treat pain and inflammation.
6. Cambendazole: It is a Fungicidal agent used to treat fungal infections.
References (Latest editions):
Heterocyclic chemistry by Raj K. Bansal.
Heterocyclic chemistry by T.L. Gilchrist.
Organic chemistry by Morrison and Boyd.
A textbook of organic chemistry - Arun Bahl. B.S. Bahl.





























Comments
Post a Comment