Synthesis, Reactions, and Medicinal Uses of Furan
Synthesis, Reactions, and Medicinal Uses of Furan
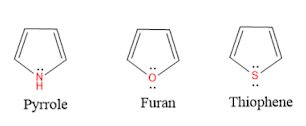
Furan is a five-membered heterocyclic compound having one Oxygen atom as a heteroatom.
Physical properties:
Colour : colorless
State : liquid
Boiling point : 31.3 oC
Melting point : -85.6 oC
Solubility : soluble in organic solvents(ethanol, ether, and acetone, etc.,)
slightly soluble in water.
Structure & Aromaticity:
All atoms in the Furan ring are sp2 hybridised, so the pyrrole contains a planar ring structure. the sp2 hybrid orbitals overlap with each other and with "s" atomic orbital of the five hydrogens forming C-C, C-O, C-H sigma bonds. all these sigma bonds lie in one plane.
Furan also has unhybridised p orbitals and these are perpendicular to the plane of the ring. each p orbital on carbon atom contains one electron and the p orbital on Oxygen contains lone pair of electrons(two electrons). the p orbital overlap to form delocalized pi molecular orbital. Furan shows aromaticity because the resulting pi molecular orbital (which contain 6 electrons) satisfies the Huckel's rule (n=1 in 4n+2).
Resonance structures:
Synthesis:
1. Paal Knorr synthesis: It is one of the most important method for the synthesis of furan. 1,4-dicarbonyl compounds undergo acid-catalyzed cyclization to give furan.
Mechanism:
2. Fiest-Benary synthesis: α-halo ketones reacts with β-ketoesters in presence of base give furan.
Mechanism:
3. From carbohydrates: Pentoses upon acid hydrolysis gives furfural. which upon treated with nickel or palladium at 200 oC gives furan.
4. From allenals: Allenals treated with Silver nitrate and methyl cyanide, cyclizes to give respective furans.
Electrophilic substitution reactions:
Electrophile attack at 2nd position gives more resonance structures than electrophile attack at the 3rd position. more resonance structures mean more stable it is.
So, electrophilic substitution reactions take place 2nd position in furan.
1. Nitration: Furan reacts with concentrated nitric acid in acetic acid or in acetic acid gives 2-nitro furan.
2. Sulphonation: Furan treated with sulfur trioxide in pyridine gives furan-2-sulfonic acid.
3. Acylation: Furan reacts with acetic anhydride and lewis acids such as boron trifluoride or tin tetrachloride give 2-Acyl pyrrole.
Oxidation:
Oxidation of furan gives succinaldehyde
Reduction:
Furan reduced with platinum and H2 give Tetrahydrofuran(THF).
Other important reactions:
Dields-Alder reaction:
Furan behaves as dienophile and reacts with various dienes to give adducts (4+2 cycloaddition reaction).
Gomberg-Bachmann reaction:
It is an Aryl-aryl coupling reaction via a diazonium salt. Furan is arylated by reacting with an aryl diazonium salt in the presence of alkali
Gattermann- Koch reaction:
In which aromatic compounds are formylated by treating with hydrogen cyanide and hydrogen chloride in presence of Lewis acid as catalyst.
Furan undergoes Gattermann-Koch reaction to form furfural.
Medicinal uses:
Drugs that contain a Furan ring:
1. Ranitidine is commonly used in the treatment of Peptic ulcer, Gastroesophageal reflux, and Zollinger-Ellision syndrome.
2. Furosemide is a Diuretic agent used to reduce excess fluid retention due to heart failure, kidney disease, and liver disease. also used in management of hypertension.
3. Ranbezolid used as an antibacterial agent.
4. Cefuroxime is a cephalosporin antibiotic used to treat pneumonia, urinary tract infections and otitis media.
5. Nitrofurantoin is an antibiotic used to treat urinary tract infections.
References (Latest editions):
Heterocyclic chemistry by Raj K. Bansal.
Heterocyclic chemistry by T.L. Gilchrist.
Organic chemistry by Morrison and Boyd.
A textbook of organic chemistry - Arun Bahl. B.S. Bahl.


































Comments
Post a Comment