Synthesis, Reactions, and Medicinal Uses of Imidazole
Synthesis, Reactions, and Medicinal Uses of Imidazole
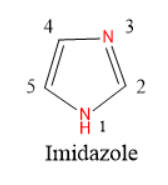
Imidazole is a five-membered unsaturated heterocyclic compound having two nitrogen atoms as heteroatoms at 1 and 3 positions.
Colour : White or colourless
State : Solid
Boiling point : 256oC
Melting point : 89 to 91oC
Solubility : Soluble in water, soluble in organic solvents
Structure & Aromaticity:
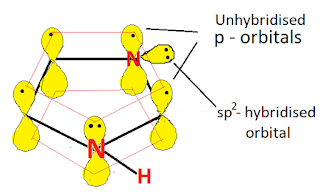
All atoms in the Imidazole ring are sp2 hybridised, so the imidazole contains a planar ring structure. the sp2 hybrid orbitals overlap with each other and with "s" atomic orbital of the four hydrogens forming C-C, C-N, C-H, and N-H sigma bonds. all these sigma bonds lie in one plane.
Imidazole also has unhybridized p orbitals and these are perpendicular to the plane of the ring. each p orbital on carbon atom contains one electron and the p orbital on nitrogen contains lone pair of electrons(two electrons). the p orbital overlap to form delocalized pi molecular orbital. Imidazole shows aromaticity because the resulting pi molecular orbital (which contain 6 electrons) satisfies the Huckel's rule (n=1 in 4n+2).
Synthesis:
1. Radiszewski synthesis: It is the most common way of synthesis of imidazole.
Dicarbonyl compound condensed with aldehyde and two molecules of ammonia gives imidazole.
2. From α-halo carbonyl compound: This reaction involves an interaction of α-halo ketone and amidine.
3. From dehydrogenation of imidazolines: Condensation of 1,2-diamine with nitriles gives imidazoline, which upon dehydrogenated with barium manganate yield imidazole.
Electrophilic substitution reactions:
Electrophilic substitution takes place more preferably at 4th position, if 4th position blocked, substitution takes place at 5th position.
1. Nitration: Imidazole upon treating with nitrating mixture gives 4-nitro-imidazole
2. Sulphonation: Imidazole reacts with Conc. Sulphuric acid in oleum gives imidazole-4-sulphonic acid.
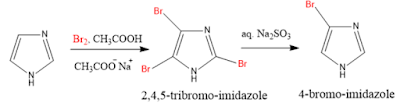
3. Halogenation: Imidazole on chlorination gives 4-Chloro-imidazole.
Other important reactions:
Protonation: Imidazole acts as a base, it accepts proton forms imidazolium cation.
N-Alkylation: Imidazole reacts with an alkyl halide gives N-Alkyl imidazolium salt, which loses N-proton by interacting with another imidazole molecule and gives N-Alkyl imidazole.
N-Alky imidazole reacts with an alkyl halide gives N-1, N-3-dialkyl-imidazolium salt.
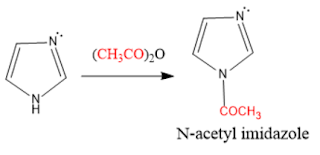
N-Acetylation: Imidazole reacts with acetic anhydride gives N-acetyl imidazole.
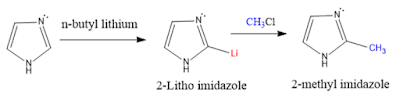
Lithiation: Imidazole reacts n-butyl lithium gives 2-litho imidazole, which upon treating with alkyl halides gives 2-alkyl imidazole.
Oxidation: Imidazole oxidises with hydrogen peroxide leads to ring-opening and gives oxalamide.
Medicinal uses:
1. Ketoconazole is an antifungal agent that is used to treat infections like athlete's foot, ringworm and seborrhea.
2. Miconazole is used to treat athlete's foot, ringworm and other fungal infections.
3. Cimetidine is used to peptic ulcer, gastro oesophagal reflux disease and heartburn.
4. Metronidazole is an antiamoebic agent used to treat amoebic dysentery.
5. Methimazole and Carbimazole are Anti-thyroid drugs used to control hyperthyroidism.
6. Albendazole and Mebendazole are Antihelmenthic agents used to treat helminthiasis.
References (Latest editions):
Heterocyclic chemistry by Raj K. Bansal.
Heterocyclic chemistry by T.L. Gilchrist.
Organic chemistry by Morrison and Boyd.
A textbook of organic chemistry - Arun Bahl. B.S. Bahl.

























Comments
Post a Comment